Spray Wires and the Spray Metal Coating
The three metal wires used for metalizing structural steel are zinc, aluminum, and the 85% zinc / 15% aluminum alloy. Coatings of these metals protect steel in three ways.
- First, the sprayed-metal is a barrier coating that separates the steel substrate from the air and moisture in the environment24 (See AWS C2.14 “Corrosion Tests of Flame-Sprayed Coated Steel” in the section Sprayed-Metal Coating Thickness regarding minimum thicknesses.)
- Second, these coating metals are anodic to steel and protect the steel galvanically.25
- Third, zinc and aluminum corrosion products develop in the pores and on the surface of sprayed-metal. This corrosion layer shields the metallic coating from the corrosive atmosphere slowing the consumption (oxidization) of the galvanic coating metal. See the reference to NAVSEA at Sealed Metal Coating below.

Spray Wires for Coating Structural Steel
The three metals used to protect steel in atmospheric or immersion service are 99.99% zinc, aluminum in the range of 99.0-99.5%, and the zinc aluminum alloy composed of 85% zinc and 15% aluminum. The following is general information on each of these sprayed-metal coatings.
Zinc (99.99%)
Metalized zinc coatings are used in rural, industrial, and marine environments. The three mechanisms of protection described above are most pronounced with zinc, and it is by these three mechanisms, barrier, galvanic action, and corrosion product development that zinc and zinc alloy coatings protect steel.
Zinc is the most active of the three coating metals and provides a high degree of cathodic protection to the underlying steel. Zinc’s corrosion resistance is unique; it has a high corrosion rate, which is offset by the formation of the aforementioned corrosion products on the metal coating’s surface.
- Sprayed zinc and 85/15 are commonly specified for metalizing bridge steel in part because of their relative ease of application and adhesive strength. This is especially important for on-site work and will be a factor when repairing previously metal-coated steel.
- Zinc’s galvanic protection of steel reaches beyond the coated surface to protect uncoated areas for example where there is mechanical damage.26
- Sprayed zinc coatings do not degrade in sunlight.
- Zinc coatings react sufficiently with concrete to form a mechanical bond, which is important when metalizing bridge expansion joints or end dams27
- Zinc metalizing is used to coat bare, partially galvanized steel girders when those girders cannot fit properly into a galvanizing kettle. Sprayed zinc is the best way to zinc coat bare, partially galvanized steel, and to repair damaged galvanized steel. (See ASTM A780 Standard Practice for Repair of Damaged and Uncoated Areas of Hot-Dip Galvanized Coatings.)28
- Cathodic protection of rebar set in concrete. Metalized zinc forms the anode in cathodic protection systems that protect rebar set in concrete e.g. the reinforcing steel in bridge substructures, parking garages, etc. There are two systems that use sprayed zinc; one relies on an impressed electrical current, and the other is passive or galvanic.29
- Zinc pH range is 6 to 1230
- The tensile adhesion strength of metalized zinc on blast cleaned steel is about 500 psi.31
- Service temperatures up to about 140° F (60° C)32
- Density: .258 lb per cubic inch
Zinc-Aluminum Alloy Wire (85/15)
The 85/15 alloy combines characteristics of zinc and aluminum. As with pure zinc, the alloy provides a high degree of galvanic protection. The 15% aluminum enhances the coatings resistance to chloride and sulfur dioxide, increases the coating’s maximum service temperature, and its hardness.
- The alloy is about 20% lighter than pure zinc. See the the alloy’s density below.
- Its tensile adhesion strength is about 700 psi on blast cleaned steel.33
- 85/15 tolerates a higher service temperature that pure zinc. Its maximum service temperature is up to about 600° F (315°) C.34
- Density: .206 lb per cubic inch. (Compare the densities of each of the three metal wires. The density of the 85/15 alloy is a function of the percent of zinc and aluminum in the alloy and the densities of each of those metals.)
Note: Coating hardness was a factor in the U.S. Army Corps of Engineers choice of 85/15 for metalizing dam gates because it withstood the abrasion of debris impacting the metalized gates.35
Aluminum
Sprayed aluminum may be used in heavily polluted industrial environments where there are very high concentrations of sulfur dioxide and other pollutants, and at elevated temperatures.
- Aluminum is said to passivate, that is, aluminum reacts with the atmosphere, and a hard, tenacious oxide film forms on the aluminum metal surface. Passivation contributes to aluminum’s resistance to SO2
- Aluminum should not be placed in direct contact with uncured alkaline concrete. 36 (Compare this with the note above about zinc’s mechanical bond with concrete.) 37 38
- From BS 5493,
For temperatures up to about 1022° F (550° C) aluminium (175 μm nominal thickness) is suitable as sprayed.
40 - Aluminum pH range is 4 to 1139
- Density: .093 lb per cubic inch
Sprayed-Metal Coating Thickness
The thicknesses of sprayed-metal coatings vary according to the spray metal used, the environment in which the coated steel will be exposed, and the required service life of the coating. For example, a structure in an “exterior exposed non-polluted inland” atmosphere may be coated with 6 mils of unsealed zinc;” 41 the interior of a potable water tank might be coated with a nominal 7 mils of zinc;42 a bridge in an “exterior exposed polluted coastal” atmosphere could be coated with as much as much as 14 mils of zinc to achieve a very long service life of forty years, or longer. [BS 5493 and CSA G189]43 44
Coating Thickness Recommendations in BS 5493, CSA G189 and ISO 2063
British Standard 5493, Table 3, Parts 1 through 10, and Canadian Standard G189, Appendix B Tables 1, and 2 give guidance on coating thickness. ISO Specification 2063 provides a “minimum local thickness chart” at Table 1, and a separate chart of “Minimum thicknesses recommended for different purposes” in Annex B, Table B.1.
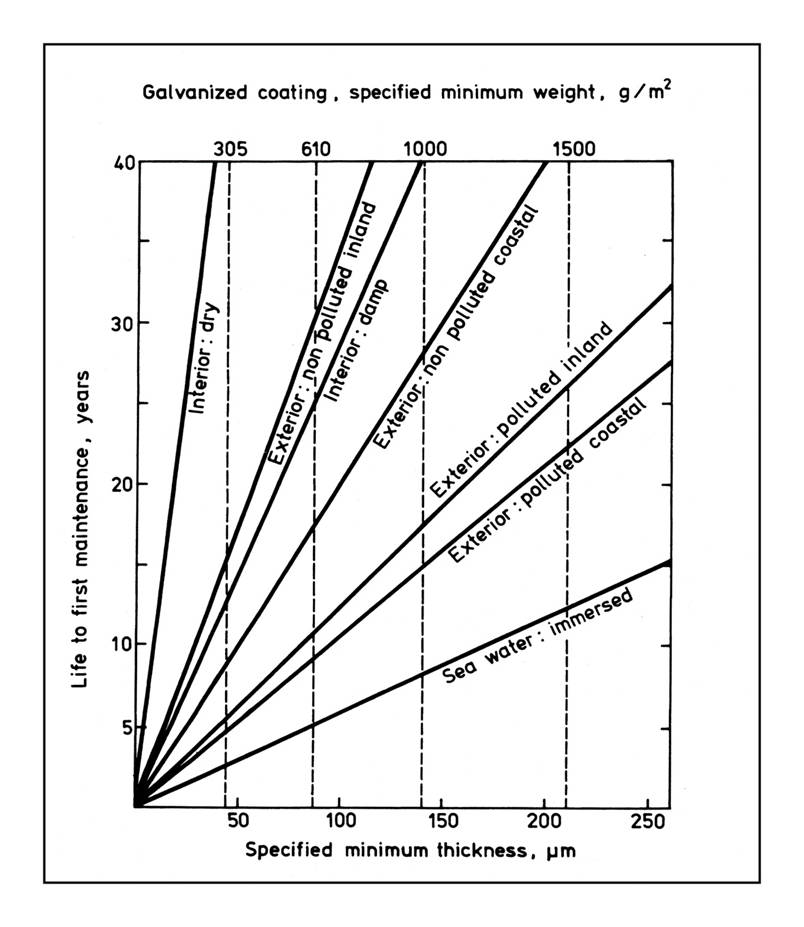
In the case of a sprayed zinc coating, the coating thickness, or more properly the weight of zinc coating, determines the life of the coating. The British Standard explains
The desirable weight or thickness of bare zinc for use in different environments can be derived from figure 1. The metal corrodes at a predictable and uniform rate, which increases as sulphur dioxide pollution of the environment increases.
45
The Standard’s Figure 1 below illustrates how the amount of zinc (thickness / weight of zinc metal) is reflected in the service life of the coating on the y-axis, in the seven different environments.46
The 19-Year Report Conclusions – Coating Thickness
Beginning in January 1953 and continuing into the 1970’s, the American Welding Society conducted a test to evaluate flame-sprayed zinc and aluminum at various test sites across the United States. In the conclusions of the 1974 report, AWS C2.14 Corrosion Tests of Flame-Sprayed Coated Steel, we read
(1) Aluminum-sprayed coatings 0.003 in. to 0.006 in. thick, both sealed and unsealed, gave complete base metal protection from corrosion in sea water and also in severe marine and industrial atmospheres.
(2) Unsealed zinc-sprayed coatings required 0.012 in. minimum thickness for complete protection in sea water for 19 years. In severe marine and industrial atmospheres, 0.009 in of unsealed zinc or 0.003 in. to 0.006 in. of sealed zinc are needed for 19-year protection. [AWS C2.14] 47
BS 5493 - Two more guidelines on coating thickness
Coating thicknesses less than 100 μm [0.004”] are not usually specified unless the sprayed-metal is to be sealed or painted immediately.48
For most atmospheric environments, there is no advantage in spraying aluminium to a thickness greater than 150 μm [0.006”] (nominal).49
Conclusion
The proof of metalizing’s effectiveness is well known; the consensus specification, the metal spraying technology, and the spray materials are all in place ready for structure owners who are searching for a coating that will provide better protection than paint.
A Comparison of Touch-up Materials for Galvanized Products, Cominco Metals,” Toronto, 1995
Specification for Thermal Spraying Zinc Anodes on Steel Reinforced Concrete, FHWA Document RD-98-088, Corrosion Protection – Concrete Bridges , Chapter 5, Section B (d) (2), United States Patent No. 4,506,485, (Apostolos) Process for Inhibiting Corrosion of Metal Embedded in Concrete and a Reinforced Construction, Mar. 26, 1985 and FLDOT Technical Special Provision, T459-6 Arc Sprayed Zinc Anode Application