The Metalizing Process
Metalizing with either zinc or aluminum is a versatile and cost-effective way of stopping steel corrosion. A metalized coating of pure zinc or an alloy of 85% zinc and 15% aluminum protects steel for a longer time than paint whether that metalized coating is applied to new steel in-shop, or applied as a maintenance coating to existing structures on-site. Because of this longer service life, metalizing’s life-cycle cost can be lower than that of paint, and life-cycle cost is the objective way to compare the cost of one coating type versus another.
Whether shop or field applied, the metalizing process includes three essential steps:
- Surface preparation
- Metal spraying
- Sealing and or topcoating
Surface Preparation
Proper surface preparation is the indispensable first step in the metalizing process. Surface preparation includes cleaning the steel (removing dirt, rust, millscale, etc.) and roughening the steel surface to create the surface profile or anchor tooth pattern to which the sprayed-metal adheres.
The blast cleaning abrasive (grit) shall be of the size and grade that will produce a uniform angular surface profile depth of 2.5 to 5 mils. In general, one can say that the rougher the surface, the higher the bond strength. The following is taken from an early version of the SSPC-CS 23.00, the forerunner of the current Joint Standard on metalizing.
The depth of surface profile required depends upon thermal sprayed coating thickness. When specifying greater than 12 mils (300 microns) of thickness, a minimum of 3 mils (75 microns) surface profile (not to exceed 1/3 total coating thickness) shall be achieved to ensure coating adhesion. NOTE: Adhesive strength tends to increase with surface roughness.
14
(See the reference to excess profile depth
at Service Life & Repair under the heading AASHTO S8.2/SSPC-PA 19 – Section 11 & Appendix A.)
SSPC Specifications SP5 White Metal (NACE 1), SP10 Near-White Metal (NACE 2), and SSPC- SP16 Brush-Off Blast define blast cleaning. For more details on surface preparation for metalizing, see AWS C2.23 (Joint Standard), Clause 5 and specifically 5.2 Surface Cleanliness, 5.2.2, guidance on abrasive selection, and 5.3 Surface Profile.
Metal-Spraying
The two methods for spraying zinc and aluminum onto structural steel are gas flame-spray and electric arc-spray; either an oxy-fuel flame or an electric arc is used to melt the zinc or aluminum spray wire(s).15 The oxy-fuel gun produces temperatures in the range of 5,000-5,600°F; the arc-spray temperature may be twice that of the gas flame and both temperatures are well above the melting points of zinc (787 °F) and aluminum (1220 °F).
The gradual galvanic consumption of the coating metal is the primary mechanism by which the sprayed zinc metal protects steel. (See Sealing & Topcoating.)
The metalizing gun feeds the spray wire(s) into its heat source, a flame, or an arc, where the tip(s) of the metal wire(s) melt. Compressed air atomizes the molten metal stripping loose particles of that metal and propelling those particles to the roughened steel surface. The metal particles impact on the steel, flatten, freeze, and build up particle upon particle to form a coating.
The sprayed-metal establishes a close mechanical bond with the steel and, unlike zinc paint, there is direct contact between the galvanic metal and the steel. The sprayed-metal coating is somewhat porous and over time corrosion products of the zinc or aluminum form in the porosity and on the surface of lamellar structured metal coating.16 These corrosion products, oxides, hydroxides, carbonates, fill voids in the coating and close any through porosity to the substrate. The buildup of oxides and other corrosion products adds another dimension to the coating by its shielding the sprayed-metal itself from the atmosphere, slowing its galvanic consumption.
Compare Metalizing’s Advantages with the disadvantages of paint coatings
Sprayed-metal contains no VOC’s. Sprayed zinc and sprayed aluminum contain no volatile organic compounds (VOC's), and require no drying or curing time.
No cure time. The sprayed-metal cools immediately on impact and is then ready to accept a liquid sealer. The complete process, blasting, metalizing, and sealing may be completed in just one day meaning that in fabricating and coating shops, workspace is freed up. On-site, scaffolding can be moved as soon as sealing is completed.
No application temperature limit. Zinc and aluminum may be applied nearly year ‘round in almost any shop or field environment. Since ambient temperature does not limit the use of the process, as is the case with painting, the coating season can be extended by several months in most regions allowing for key bridges, water tanks and other structures to be coated on-site when demands on those facilities are low.17 (See the Joint Standard, paragraphs 5.1.1 “Ambient Temperature” regarding dew point, and 8.3 “Spraying in Low Temperature Environments” regarding condensation. Also, refer to the additional coating temperature information in the galvanizing information below.)
Very long service life. According to two national standards, British Standard 5493 and Canadian Standard G189, metalized coatings provide very long service lives even in the most corrosive environments.18 In the British Standard for instance, only sealed metalized coatings are said to provide a very long service life in “Sea water splash” or “Sea water immersed” service. Not one of the dozen or so paints types listed in 5493 is ranked in the very long service life category in those two environments. The Canadian Standard proposes metalizing service lives of 40 years or longer in industrial and marine environments.19
And compare metalizing with the disadvantages of galvanizing
Galvanizing is by
Hot-Dip
Immersion. Metallic zinc coatings may be applied to structural steel by dipping steel into a kettle of molten zinc or by spraying molten zinc onto cold steel. Both coatings work, but they are not equal. Hot dip galvanizing can affect the metallurgy of the steel whereas metalizing, a relatively cool process, does not damage the steel fabrication or its welds.Metalizing is a cool process.Metalizing guns produces temperatures in the thousands of degrees Fahrenheit that melt the spray wire(s), however, the steel target’s surface temperature does not exceed about 250-300° F. At these temperatures, steel does not deform, there are no adverse effects on the metallurgical properties of the steel, and metal spraying does not damage welds. 20 These heat induced problems, deformation, metallurgical changes and weld damage can, and do happen when steel is immersed in 850°F molten zinc.
Painting galvanized steel is not simple and not always reliable. In some circumstances, zinc coated steel must be painted for aesthetic or other reasons. A hot-dip galvanized surface is relatively smooth. It is well known that painting galvanized steel is complicated requiring extensive surface preparation, which, if not done correctly, will lead to early paint failure. On the other hand, sealed-sprayed-zinc has a slightly rough texture similar to blast cleaned steel and is an excellent surface for paint.21
Heavy galvanized coatings. The sprayed-metal coating is accurately and consistently applied at a specified thickness. Specifications for hot-dip galvanizing do not set maximum coating thicknesses sometimes resulting in excessive zinc thickness and coating imperfections such as drip lines and spikes that require special manual prep before painting. These too thick hot-dip coatings can also be subject to stresses during cool down from the 850°F galvanizing kettle temperature.22 Incidentally, the excess zinc will also add to the per pound galvanizing charge.
Coating thickness and coating uniformity affect the life and effectiveness of zinc coatings.
Selective application. The sprayed-metal coating can be applied to selected areas of the steel leaving uncoated those areas that are to be left bare by design. This is not possible with galvanizing.
Large structural members. The largest structural girders may be metalized whereas the galvanizing kettle length limits the size of girders that can be routinely zinc coated by that process. (In some cases, metalizing is used by galvanizers to supplement and to repair galvanized coatings. See ASTM A780 Standard Practice for Repair of Damaged and Uncoated Areas of Hot-Dip Galvanized Coatings.)
Range of steel grades. All grades of steel and wrought iron can metalized. This is not the case with galvanizing. 23
The sprayed-metal coating may be applied anywhere. Metalizing can be applied at the fabricator, in a specialized metalizing shop, or at the job site meaning that a construction project is not burdened by having to ship steel to a galvanizing facility.
Coatings of aluminum and the zinc-aluminum alloy (85% zinc and 15% aluminum) are not available by hot dip galvanizing. These metalizing materials offer some benefits for coating steel that will be exposed in marine and industrial environments, and at elevated temperatures.
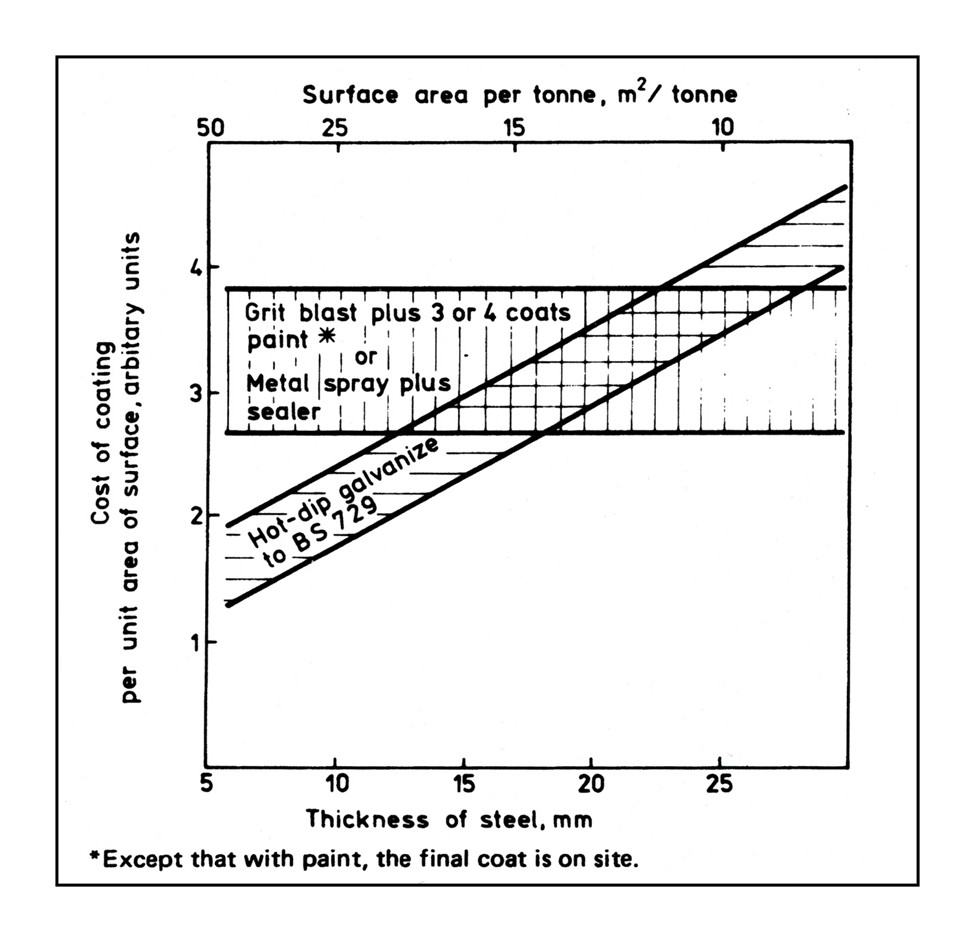
Metalizing can be more economical than galvanizing when the surface area/weight ratio is low. See the BS 5493 graph below. Galvanizing becomes more expensive as the surface area to weight ratio decreases (the upward slope). In other words, metalize large, heavy, thick girders.
Opinion
Owners choose to metalize because it provides long term protection for steel. On the other hand, the primary reason for awarding a structural steel painting contract is a low-bid price.
It is worth repeating: More zinc can be deposited by metalizing than by painting, and the sprayed-metal coating is more uniformly applied than that deposited by hot-dip galvanizing. Both the weight of zinc deposited and the uniformity of the coating affect the coating’s service life.
lamellardescribes the coating structure. Lamellar refers to a thin plate or scale-like structure; a build-up of lamellae (plates) illustrates the metalized coating structure.